Abstract
Introduction: Novel therapies for relapsed/refractory multiple myeloma (RRMM) have demonstrated durable responses that prolong survival, thus assessing health-related quality of life (HRQoL) is becoming increasingly important in patient (pt) care. Long-term RRMM treatment may lead to a higher cumulative symptom burden and negatively affect HRQoL. Indeed, the effect of treatment on HRQoL is reduced in pts with RRMM on later vs initial lines of therapy (LoTs) (Nielsen et al, Eur J Hematol 2017). Consequently, there is a need for treatments that demonstrate durable efficacy while preserving HRQoL. Pomalidomide plus dexamethasone (Pd) has been shown to be an effective treatment for RRMM that does not negatively affect HRQoL in later LoTs (Song et al, Haematologica 2015). In the randomized phase 2 ELOQUENT-3 study (NCT02654132), addition of the SLAMF7-targeted monoclonal antibody elotuzumab (elo) to Pd (EPd) showed a 46% reduction in the risk of progression/death vs Pd, and acceptable safety in pts with refractory or relapsed and refractory MM for whom therapy with lenalidomide and a proteasome inhibitor had failed (Dimopoulos et al, EHA 2018 [LB2606]). We present pt-reported outcome (PRO) data to assess the impact of EPd on HRQoL in pts from ELOQUENT-3.
Methods: The M.D. Anderson Symptom Inventory MM module (MDASI-MM) and the 3-level version of the EuroQoL 5 Dimensions Questionnaire (EQ-5D-3L), which included the global health visual analog scale (VAS) measure, were administered at baseline (BL) and at the start of every 28-day treatment cycle until discontinuation and at survival follow-up. All randomized pts with BL and ≥1 post-BL assessment were included in the PRO analysis. Changes from BL scores were evaluated descriptively: high scores indicate better health for EQ-5D-3L, but more severe symptoms for MDASI-MM. Time to first deterioration was evaluated, with a deterioration event defined as the absolute value of the change from BL ≥ the responder definition threshold (MDASI-MM symptom item assessing pain, fatigue, and bone aches: 2; EQ-5D-3L VAS: 7).
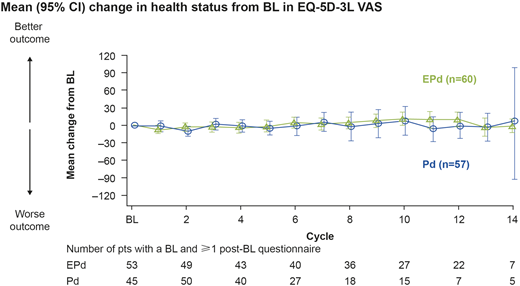
Results: This analysis included 117 randomized pts (EPd, n=60; Pd, n=57) who had received a median of 3 prior LoTs. PRO completion rates at BL were 79% for MDASI-MM (EPd, 85%; Pd, 72%) and 84% for EQ-5D-3L (EPd, 88%; Pd, 79%). Although completion rates between treatment groups were similar throughout the study, analysis of QoL was not feasible after Cycles 13 for EPd and 10 for Pd as the number of pts with questionnaires dropped below 20%. Mean BL QoL scores were similar for EPd vs Pd (EQ-5D-3L utility, 0.676 vs 0.682; EQ-5D-3L VAS global health status, 61.9 vs 62.9). A clinically meaningful deterioration from BL in EQ-5D-3L VAS health status (mean change -9.0) was observed with Pd at Cycle 2. In contrast, there were no clinically relevant deteriorations in EQ-5D-3L VAS health status with EPd, and a sustained improvement from BL was observed between Cycles 9 and 12 (mean change 8.2, 11.7, 9.7, and 10.4, respectively; Figure). MDASI-MM symptom scores for pain and bone aches showed no clinically relevant change from BL levels in both groups, but fatigue worsened (mean change 2.5) in the Pd group at Cycle 9. Pts who discontinued treatment early tended to have lower HRQoL in both treatment arms. There were no differences in time to first deterioration for EPd vs Pd for MDASI-MM symptoms pain, fatigue, or bone aches, or EQ-5D-3L and VAS domains. Although the risk of deterioration in the severity of MDASI-MM core symptoms (symptoms common across cancer types and treatments) was reduced by 17% (hazard ratio 0.83; 95% CI 0.49-1.42) for those receiving EPd vs Pd, this was not statistically significant (p=0.618); the median time to deterioration was 3.8 mo with EPd and 1.2 mo with Pd.
Conclusions: In ELOQUENT-3, pt-reported health status was maintained in pts who remained on EPd per EQ-5D-3L VAS, with no worsening of pain, fatigue, or bone aches per MDASI-MM. There were minimal differences in QoL between pts who received EPd and Pd, suggesting that the addition of elo to Pd does not impair HRQoL, although HRQoL may have been overestimated due to the small sample and treatment discontinuation. These pt-reported findings complement strong clinical data that demonstrated clinically relevant improvements in efficacy and a favorable safety profile for EPd, further supporting the use of this regimen in RRMM.
Study support: BMS. Writing support: Simon Wigfield, Caudex, funded by BMS.
Weisel:Amgen, BMS, Celgene, Janssen, and Takeda: Honoraria; Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen, Celgene, Janssen, and Sanofi: Research Funding. Paner:Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria. Taylor:Adelphi Values: Employment; Bristol-Myers Squibb: Consultancy. Cocks:Adelphi Values: Employment; Amgen: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Endomag Ltd.: Consultancy. Espensen:Adelphi Values: Employment; Bristol-Myers Squibb: Consultancy, Other: I am an employee of Adelphi Values, a consulting firm that has received payment from BMS for statistical data analysis in BMS trials. Popa-McKiver:Bristol-Myers Squibb: Employment. Chen:Bristol-Myers Squibb: Employment. Cavo:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal